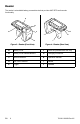
SurgiCount®+ System Instructions For Use Cradle 0694-002-002 Reader 0694-002-005 Stand 0694-002-001 Alternative Cradle Mounts 0694-002-003 0694-002-004 Disposables 0694-002-010 0694-002-006 EN 700001138880 Rev-AC 2021-02
Table of Contents Important Information .................................................................................... 2 Using this Manual ....................................................................................... 2 Contact Information..................................................................................... 2 Intended Users .......................................................................................... 2 Indications For Use ..........................................
Important Information Using this Manual This manual is the most comprehensive source of information for the safe, effective, and compliant use and/or maintenance of your product. This product is intended for use by trained and experienced healthcare professionals only. Read and understand this manual before using the product or any component compatible with the product. Contact Stryker for training as needed. This manual is a permanent part of the product. Keep this manual for future reference.
For Use With WARNING • Use only Stryker-approved electronic components and accessories. Failure to comply may result in degraded performance, increased electromagnetic emissions or decreased electromagnetic immunity of the system, see Electromagnetic Compatibility (page 25). • Use only Stryker-approved components and accessories, unless otherwise specified.
Safety Directives Gen era l Safety WARNING • Only healthcare professionals that are trained and experienced in the use of this medical device should operate this equipment, see Intended Users (page 2). • Equipment is limited to professional use within a professional healthcare environment. • Healthcare professionals should be thoroughly familiar with the instructions for use, handling characteristics, and the indicated, contraindicated, and intended uses of this equipment.
Radio Frequency Exposure and Magnetic Resonance (MR) Safety WARNING • Always maintain a minimum separation distance of 9 inches [23 cm] from the head and torso of a person and the UHF RFID antenna of the SurgiCount®+ Reader while in use. Failure to comply may cause FCC RF exposure limits to be exceeded. • The SurgiCount®+ System is MR unsafe. Do not use the system in an MR environment.
Definitions N o t e - Additional product and/or labeling symbols are defined in the Symbol Definition Chart supplied with the product.
Product Overview Cradle The cradle facilitates communication between the tablet and the reader. Additionally, the cradle provides battery backup power to the system when disconnected from electrical power.
Reader The reader is a handheld battery powered device that provides UHF RFID and barcode functionality.
Stand (Recommended Cradle Mounting Option) N o t e - The stand is the recommended cradle mounting option for the SurgiCount®+ System.
Alternative Cradle Mounting Options Fi gure 6 – Wa ll Mou nt EN 10 Figu re 7 – Pole Mount 700001138880 Rev-AC
Getting Started Before Use 1. Remove all the components from packaging material. 2. Inspect equipment for damage, see Inspection and Maintenance (page 19). W A R N I N G - When installing the cradle to a mounting option, always follow the installation guide provided with the equipment. Failure to comply may result in healthcare staff injury. 3. [Facility Maintenance Staff] – Refer to the applicable installation guide and complete the cradle installation, see Documentation (page 3). 4.
Using Your Product Charging the System C A U T I O N - Do not use the USB port to charge the reader. Failure to comply may result in damage to the device. Note • When connected to electrical power, the system will take approximately five (5) hours to fully charge. It is recommended to fully charge the system before each use. • When disconnected from electrical power, a fully charged system will provide approximately ten (10) hours of use.
Reader Instructions • Docking and Undocking the Reader (page 13). • Replacing the Reader Battery (page 14). • Preparing the Reader for Sterile Use (page 16). Docking and Undocking the Reader Dock the Reader 1. Rotate the reader and grasp the front of the device. 2. Insert the reader into the cradle (Figure 10). Undock the Reader 1. Grasp the front of the reader. 2. Pull the reader out of the cradle (Figure 10). 3. Rotate the reader and grasp the handle of the device.
Replacing the Reader Battery 1. Remove the Reader Battery Cover (page 14). 2. Remove the Reader Battery (page 15). 3. Install the Reader Battery (page 15). 4. Install the Reader Battery Cover (page 15). Remove the Reader Battery Cover 1. Press and hold the battery cover release buttons (Figure 11). 2. Slide the cover back and remove (Figure 12).
Remove the Reader Battery 1. Press in on the battery (Figure 13). 2. Remove the battery (Figure 14). Figure 13 – Press in on the Battery Fi gure 14 – Re move th e Battery Insta ll the Read er Ba ttery 1. Align and insert the battery tabs into the slots in the battery compartment (Figure 15). 2. Press down on the battery (Figure 16). Fi gure 15 – Align a nd Inse rt the Battery Fig ure 16 – Press Down on the Batte ry Insta ll the Read er Ba ttery Co ver 1. Align the battery cover over the battery. 2.
Preparing the Reader for Sterile Use 1. [Circulating Nurse] – Open the reader cover sterile packaging. 2. [Circulating Nurse] – Aseptically transfer the reader cover to the sterile field. 3. [Scrub Technician] – Unfold the closed end of reader cover. 4. [Scrub Technician] – Place hands inside the reader cover cuff to create an opening. 5. [Circulating Nurse] – Aseptically transfer the reader into the opening of reader cover. 6.
Interacting with the Stand W A R N I N G - Always remove used sponge bags and fold the arms inward prior to transporting or positioning the stand on inclined surfaces. Figu re 18 – Unfol ding th e Arms Fi gure 19 – Ha nging Sponge Bags Fig ure 20 – Loadin g Disposables CAUTI ON • Always ensure the power cord is properly wrapped prior to transporting. • Always lock the casters when the stand is stationary, and unlock prior to transporting.
After Use Cleaning and Disinfection WARNING • Always clean and disinfect the equipment as indicated upon initial receipt and before each use. Failure to comply may cause infection and result in patient or healthcare staff injury. • Always consult the instructions for use that accompanies accessories for product specific cleaning requirements. CAUTI ON • Do not immerse the equipment in liquid. • Do not allow liquids or moisture to enter any electrical connection. • Do not sterilize the equipment.
References Inspection and Maintenance WARNING • Upon initial receipt and before each use, inspect equipment for damage. Do not use any equipment if damage is apparent or the inspection criteria are not met. • Do not disassemble, modify, service, or repair any equipment without the authorization of the manufacturer. Failure to comply may result in electric shock or fire. For assistance, contact Stryker.
• To expedite returns, always include the following information with the returned equipment: • Contact name, address, phone number • Repair purchase order number • Part and serial number(s) • Detailed reason for return Troubleshooting W A R N I N G - Do not disassemble, modify, service, or repair any equipment without the authorization of Stryker. Failure to comply may result in electric shock or fire. For assistance, contact Stryker.
Observation Tablet does not recognize the cradle. Cause Co rrective Acti on Tablet battery is at end of life. Contact Stryker. Cradle is damaged. Contact Stryker. Tablet is not fully docked. Verify the tablet is properly docked, see Interacting with the Cradle (page 12). Cradle is damaged. Contact Stryker. Table 2 – Reader Troubleshooting Observation Cause Co rrective Acti on Reader is not charging. Reader is not fully docked.
Specifications Surg iCou nt+ Crad le REF: 0694-002-002 Dimensions: W i d t h : 8.4 in H e i g h t : 13.6 in D e p t h : 8.6 in Weight: 5.5 lbs Ingress Prote ction: IPX0 Equipment Type: Class II equipment with functional earth Internal Power: Li-ion 14.4 VDC External Power: 120VAC~60Hz, 0.9A Mode of Operation: Continuous Fre quency of Operation: 13.56 MHz RF Bandwidth: 13.553 MHz – 13.567 MHz Modulation: ASK (Amplitude Shift Keying) RF Field Strength: 44.
Surg iCou nt+ Reader REF: 0694-002-005 Dimensions: W i d t h : 3.1 in H e i g h t : 5.1 in D e p t h : 7.3 in Weight: 0.96 lbs Ingress Prote ction: IPX0 Equipment Type: Class I equipment Internal Power: Li-ion 4.2 VDC External Power: 5 VDC Mode of Operation: Continuous UHF RFID F r e q u e n c y o f O p e r a t i o n : 902.75 MHz – 927.25 MHz P e a k O u t p u t P o w e r : 981.
Surg iCou nt+ Stand REF: 0694-002-001 Dimensions: W i d t h : 24.0 in H e i g h t : 70.8 in D e p t h : 24.0 in Weight: 52.
Electromagnetic Compatibility WARNING • Avoid locating equipment adjacent to the SurgiCount®+ System. If such a configuration is necessary, verify normal operation of both the SurgiCount®+ System and the adjacent equipment during use. • Always maintain a minimum separation distance of 6 inches [15 cm] from an in-use pacemaker and the UHF RFID antenna of the SurgiCount®+ Reader while in use. Failure to comply may cause interference with the pacemaker.
Guidance and Manufacturer’s Declaration - Electromagnetic Immunity This product is intended for use in the electromagnetic environment specified below. The customer or the user of this product should assure that it is used in such an environment.
Guidance and Manufacturer’s Declaration - Electromagnetic Immunity Conducted RF IEC 61000-4-6 Radiated RF IEC 61000-4-3 3 Vrms150 kHz to 80 MHz outside ISM bands 80% AM at 1 kHz 3 Vrms150 kHz to 80 MHz outside ISM bands 80% AM at 1 kHz 6 Vrms150 kHz to 80 MHz in ISM bands 80% AM at 1 kHz 6 Vrms150 kHz to 80 MHz in ISM bands 80% AM at 1 kHz 3 V/m 80 MHz to 2.7 GHz 80% AM at 1 kHz 3 V/m 80 MHz to 2.
Stryker Corporation or its divisions or other corporate affiliated entities own, use or have applied for the following trademarks or service marks: t h e S t r y k e r l o g o . All other trademarks are trademarks of their respective owners or holders.