Instruction Manual
Steriflow by Jordan Valve
3170 Wasson Road • Cincinnati, OH 45209
513.533.5600 • 800.543.7311 • 513.871.0105 (f)
steriflow@richardsind.com • www.steriflowvalve.com
Mark 901 Series
Cavity-Filled Three Piece Ball Valves
The Mark 901 Series is a sanitary ball valve
designed specically for Pharmaceutical and
food and beverage applications. It has cavity
ller seats and Tri-clamp ends to minimize where
media can collect. The inner diameter of the end
connections is the same as the ID of the ball,
ensuring no obstruction of ow from inlet to outlet.
Features
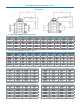
• In sizes from 1/2" (DN15) through 4" (DN100)
with a true bore design, to provide unobstructed
ow and better drainage
• Pressure ratings up to 1000 psi (69 bar) for
1/2" - 2" and 800 psi (44 bar) for 2-1/2" - 4"
• PTFE cavity ller seats
• Internal nish 20 Ra
• Lockable handle prevents accidental opening or
closing of valve
• ISO 5211 actuator mounting ange
• Designed for simple maintenance with in-line
repairable feature
• Anti-blowout stem
• Live-loaded stem seal – no threads in packing
chamber
• No sharp internal llet radius – for smooth nish
• Tri-clamp or tube weld ends
• Available as manual or actuated (pneumatic/
electric) valve
IndustrIes and applIcatIons
The Mark 901 is designed for applications in
the following industries and applications:
• Healthcare/Cosmetics
• Pharmaceutical, Bio-Pharmaceutical
- Clean Steam
- Process Gases (N
2
, CO
2
)
- Clean Dry Air and Gas
• Food & Beverage
• Specialty Chemical
• Viscous Media
MaterIal and FInIsh specIFIcatIons
• Body/End Connections/Ball:
ASTM A351 CF3M 316L SS
• Stem: ASTM A276 316 SS
• External Hardware: AISI 304 SS
• Cavity lled Seat/Packing/Thrust Washer/
Gasket: PFTE TF1641 (FDA and USP
Class VI approved resin)
• Wetted Surface Finish: ASME BPE SF1
(20 Ra µin (0,5 Ra µm) mechanical polish)