Product Manual
DOCK CLEANING
Sekisui Diagnostics recommends cleaning the Dock each day it is used.
Procedure:
Clean the Silaris Dock and surrounding area according to the instructions provided in the cleaning section of the Silaris Dock
Operator's Guide.
LIMITATIONS
• The performance of the Silaris Influenza A&B Test was determined using the procedures provided in this instructions for use. Failure
to follow these procedures may alter test performance.
• The Silaris Influenza A&B Test is for use with nasal swab specimens only.
• Improper collection, storage or transport of specimens may lead to false negative results.
• Test results should be interpreted in conjunction with the patient’s medical history, clinical signs and symptoms, and the results of
other diagnostic tests performed.
• As with other tests, negative results do not rule out Flu A or Flu B infections and should not be used as the sole basis for patient
management decisions.
• This is a qualitative test. Test line intensity is not indicative of the quantity of virus in the sample.
• Positive and negative predictive values are dependent upon prevalence. Test performance was established for the 2016-2017
influenza season. Performance may vary depending on the prevalence and population tested.
• False negative results may occur if viruses are present at levels below the test’s limit of detection.
• False negative results may occur if mutations are present in the regions targeted by the test.
• Test performance has not been evaluated for patients without signs and symptoms of influenza infection.
• Cross-reactivity with respiratory tract organisms other than those listed in the Analytical Specificity Study may lead to
erroneous results.
• Test performance has not been evaluated for the purpose of monitoring antiviral treatment.
• Test performance has not been evaluated in immunocompromised patients.
• Test performance has not been evaluated in patients who received inhaled influenza vaccine.
• This test cannot rule out diseases caused by other viral or bacterial agents.
• Analyte targets (viral nucleic acid) may persist in vivo, independent of virus viability. Detection of analyte targets does not imply that
the corresponding viruses are infectious, or are the causative agents for clinical symptoms.
• The presence of inhibitors in the sample can lead to invalid results.
EXPECTED VALUES
The prevalence of influenza varies from year to year, with outbreaks occurring during the fall and winter months. The influenza
positivity rate is dependent upon many factors, including specimen collection, test method, and geographic location. Prevalence varies
throughout the flu season and from location to location.
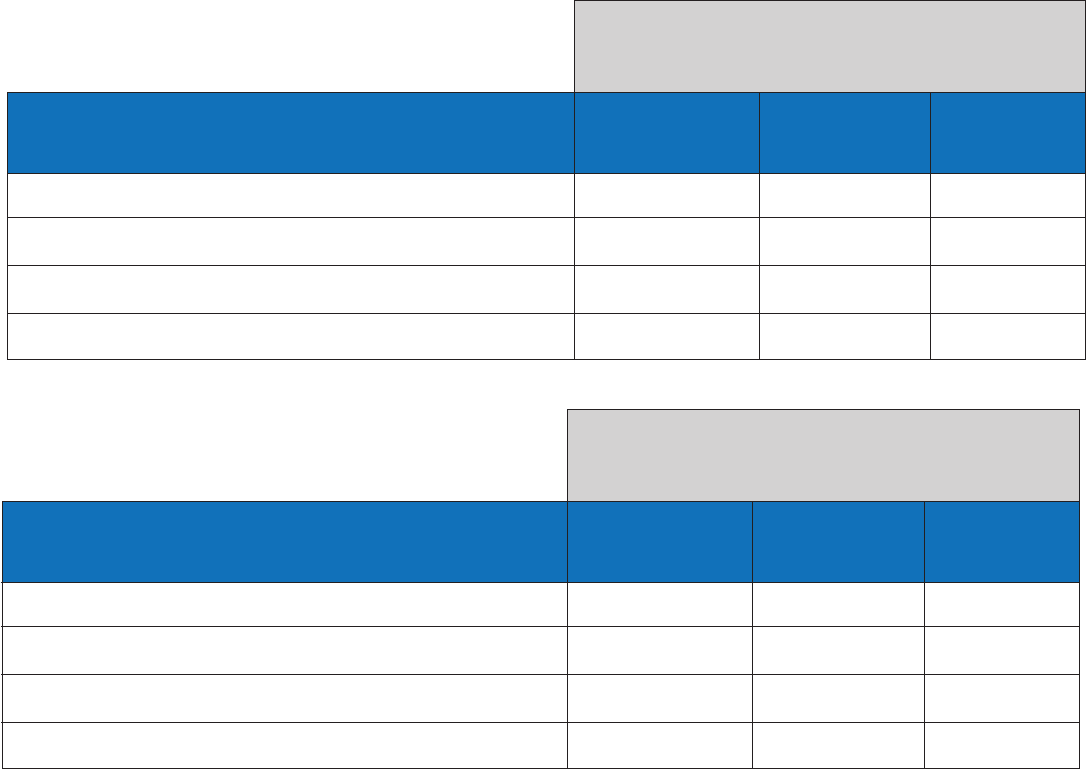
The clinical study was conducted during the 2016-2017 influenza season. The following tables show the prevalence of
influenza A and influenza B observed in three subject age categories in that clinical study.
Prospective Clinical Study during the
2016/2017 Influenza Season
Age Group
≤ 5 Years of Age 488 97 19.9%
6 to 21 Years of Age 601 172 28.6%
≥ 22 Years of Age 169 20 11.8%
Total 1258 289 23.0%
Number of
Nasal Swab
Specimens
Number of
Influenza A
Positives
Influenza A
Positivity Rate
Prospective Clinical Study during the
2016/2017 Influenza Season
Age Group
≤ 5 Years of Age 488 27 5.5%
6 to 21 Years of Age 601 91 15.1%
≥ 22 Years of Age 169 8 4.7%
Total 1258 126 10.0%
Number of
Nasal Swab
Specimens
Number of
Influenza B
Positives
Influenza B
Positivity Rate
11
12










