Product Manual
MATERIALS PROVIDED SEPARATELY
Silaris Dock (Catalog # 1026)
Silaris Influenza A&B Control Kit (Catalog # 1024)
STORAGE AND HANDLING
• Store reagents at room temperature (15°C - 30°C, 59°F - 86°F). Do not refrigerate or freeze.
• Do not reuse kit contents: Rayon Swabs, Test Cassettes, Transfer Pipettes, Control Swabs, or Nasal Swab Buffer.
• Do not remove the Test Cassette from the foil pouch until immediately before use.
• Do not use kit or reagents past the expiration date.
PRECAUTIONS
• For in vitro diagnostic use.
• Federal Law restricts sale of this device to or on the order of a licensed practitioner.
• To be used in conjunction with the Silaris Dock.
• Follow universal precautions when handling patient samples. All patient samples should be treated as if potentially infectious.
Follow standard BSL-2 guidelines when working with patient samples. Put on the appropriate personal protective equipment.
• Inactivated and lypohilized viruses are used to make the control swabs. However, control swabs, patient samples and used
cassettes should be handled as though they could transmit disease. Observe established precautions against microbial hazards
during use and disposal.
• Dispose of kit reagents and patient samples according to all local, state and federal regulations.
• Do not use Swabs or Nasal Swab Buffer other than those provided with the Silaris Influenza A&B Test kit.
• Do not write on the Test Cassette except in the indicated area on the Test Cassette label for recording sample identification and
test date.
• Do not remove the foil tab from the Test Cassette until immediately before use. Once the tab is removed, add sample immediately
and start testing.
• Once sample is added and the Dock lid is closed, the test has started. Do not move the Dock, open the lid, or unplug the Dock
until the Dock indicates the test has completed.
• Do not use any damaged kit contents.
• Do not use kit components after their expiration date.
• Sample collection and handling procedures require specific training and guidance.
• All test kit components are single use items. Do not use with multiple specimens.
• To help obtain accurate results, follow all instructions and heed all precautions in this Instructions For Use.
• Inadequate or inappropriate sample collection, handling, processing, and/or storage can yield inaccurate results.
• Use only the fixed volume Transfer Pipette provided in the kit to transfer the patient sample from the Silaris Nasal Swab Buffer
tube into the Test Cassette port. Do not pour the patient sample from the Silaris Nasal Swab Buffer vial into the Test Cassette
sample port.
• Do not use visually bloody or overly viscous samples.
• When transferring the prepared patient sample, avoid drawing up large particulates, which may clog the Transfer Pipette.
• Due to the high sensitivity of the Silaris Influenza A&B Test, contamination of the work area with previous samples may cause
false positive results. Clean the Silaris Dock and surrounding surfaces as described in the procedure in the Silaris Dock
Operators Guide.
• Do not attempt to open a used Test Cassette or a Test Cassette with closed sample port.
• Do not touch the heads of the Control Swabs. Cross contamination may occur due to the high sensitivity of the test.
• If infection with a novel influenza A virus is suspected, samples should be collected with appropriate infection control precautions
for novel virulent influenza viruses. Follow the current clinical and epidemiological screening criteria recommended by public health
authorities on whether to send the sample to state or local health departments for testing. Viral culture should not be attempted in
these cases unless a BSL-3+ facility is available to receive and culture the samples.
• Use the Results Interpretation table in this Instructions For Use to interpret results accurately.
QUALITY CONTROL
Process Controls
Each Silaris Influenza A&B Test Cassette contains two internal process controls: an internal positive control (labeled ‘C’ on the Test
Cassette) and negative control (labeled ‘NC’ on the Test Cassette). The positive process control is a non-infectious RNA bacteriophage
in the Test Cassette and is used as the positive process control to verify all assay steps (RNA extraction, reverse transcription,
amplification and detection) were executed properly. A non-influenza nucleic acid target is used as a negative control for false positive
results due to nonspecific binding.
Refer to the Interpretation of Results section of this package insert for instructions on interpreting the results for the Process Controls.
External Positive and Negative Controls:
External controls may be used to show that the Silaris Influenza A&B Test is working properly.
The Silaris Influenza A&B Test kit contains two Control Swabs:
• 1 FLU A Positive/FLU B Negative swab (yellow shaft)
• 1 FLU B Positive/FLU A Negative swab (blue shaft)
Sekisui Diagnostics recommends that an Influenza A positive/Flu B negative and an Influenza B positive/Flu A negative control be run:
• Once for each new lot or shipment of kits received
• Once for each new operator
• As deemed additionally necessary in order to conform with your internal quality control procedures, with local, state and/or federal
regulations, or accrediting groups.
Additional Silaris Influenza A&B Control Swabs may be purchased from Sekisui Diagnostics (Catalog # 1024). Run control swabs using
the same procedure as for a patient specimen.
If External QC testing fails, repeat the test using a new Control swab, reagent and test cassette or contact Sekisui Diagnostics
Technical Support for assistance at 800-332-1042 (U.S. Only) or 781-652-7800 (outside the US)., before testing patient samples.
SPECIMEN COLLECTION AND HANDLING
Proper sample collection is an important step for an accurate test result. Carefully follow the instructions below.
Nasal Swab Sample
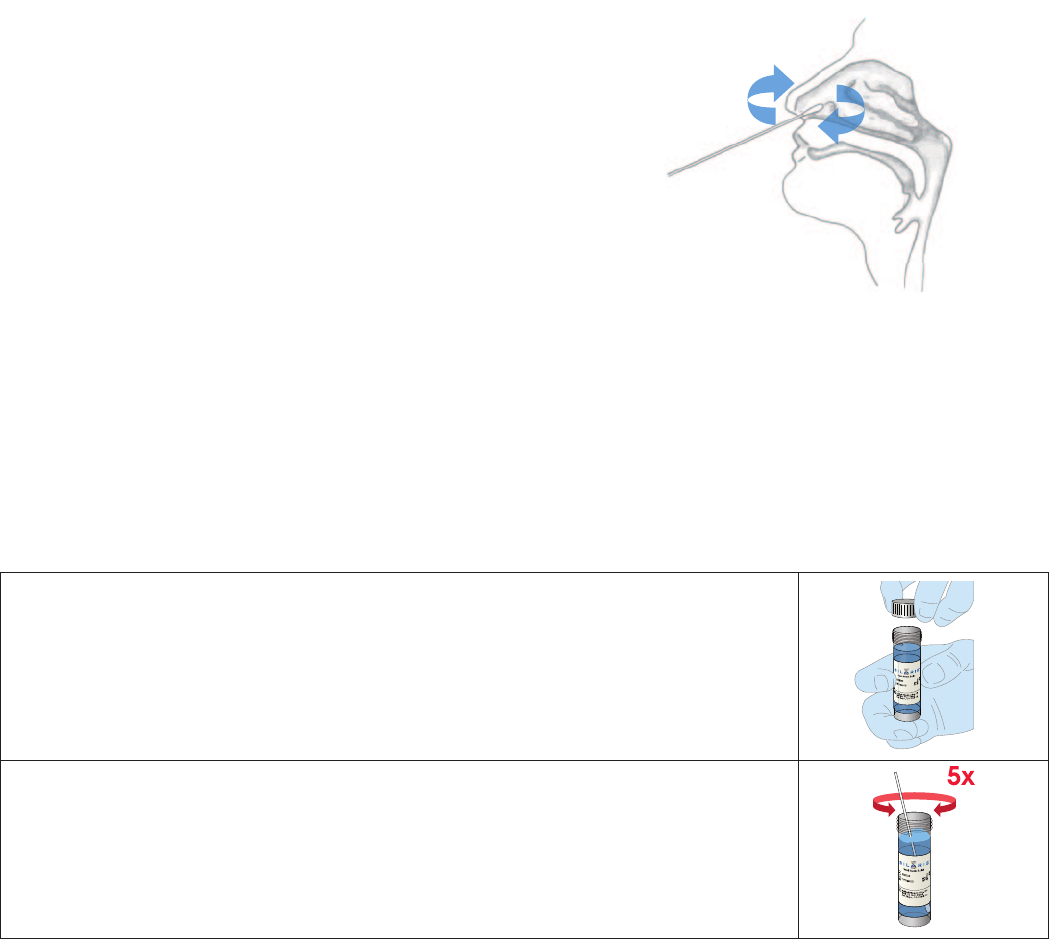
NOTE: Use only the Rayon Swabs supplied with the kit
To collect the nasal swab sample, insert the Rayon Swab into the nostril exhibiting the
most secretions. Carefully insert the swab approximately 1 inch into the patient’s nostril.
Gently rotate the swab several times against the nasal wall.
SAMPLE STORAGE AND SAMPLE EXTRACTION
For best results, direct nasal swabs should be tested immediately after collection. If immediate testing is not possible, a direct nasal
swab can be stored in its original packaging at room temperature (15°C - 30°C, 59°F to 86°F) for up to 2 hours prior to testing. If a
direct nasal swab cannot be tested within 2 hours, it can be refrigerated at 2°C - 8°C and tested within 24 hours from the time of
collection.
• Do not freeze the prepared sample prior to testing.
• The prepared sample may be stored at room temperature (15°C - 30°C, 59°F to 86°F) for up to 1 hour.
• Patient nasal swabs previously stored in viral transport media are not recommended and will invalidate the test.
Remove the cap from the Nasal Swab Buffer vial and set it aside.
Insert the nasal swab specimen into the Nasal Swab Buffer and rotate it 5 times
rubbing it against the wall of the vial.
3 4










