Product Manual
IVD
1027
CLIA WAIVED: For use with Nasal Swabs
CLIA COMPLEXITY: WAIVED.
A Certificate of Waiver is required to perform this test in a CLIA Waived environment. To obtain CLIA waiver information and
a Certificate of Waiver, contact your state health department. Additional information is available at www.cms.hhs.gov/CLIA.
INTENDED USE
The Silaris™ Influenza A&B Test performed on the Silaris Dock is a molecular in vitro diagnostic test utilizing polymerase
chain reaction (PCR) and lateral flow technology for the qualitative, visual detection and differentiation of influenza A and
influenza B viral RNA. The Silaris Influenza A&B Test uses a nasal swab specimen collected from patients with signs and
symptoms of respiratory infection. Silaris Influenza A&B assay is intended as an aid in the diagnosis of influenza infections
in conjunction with clinical and epidemiological risk factors. The assay is not intended to detect the presence of influenza
C virus.
Negative results do not preclude influenza virus infection and should not be used as the sole basis for treatment or other
patient management decisions.
Performance characteristics for influenza A were established during the 2016-2017 influenza season. When other
influenza A viruses are emerging, performance characteristics may vary.
If infection with a novel influenza A virus is suspected based on current clinical and epidemiological screening criteria
recommended by public health authorities, specimens should be collected with appropriate infection control precautions for
novel virulent influenza viruses and sent to state or local health department for testing. Viral culture should not be attempted
in these cases unless a BSL-3+ facility is available to receive and culture specimens.
SUMMARY AND EXPLANATION
Along with the common cold, influenza is one of the most common acute respiratory infections. It produces symptoms such
as headache, chills, dry cough, body aches and fever. It produces symptoms such as headache, chills, dry cough, body
aches and fever. It affects 10% – 20% of the United States population annually, resulting in more than 110,000 hospitaliza-
tions and 10,000 to 40,000 deaths.
1
The influenza A virus is typically more prevalent and is associated with more serious influenza epidemics.
2
Influenza B
infections usually present with milder symptoms.
3
Diagnosis of influenza and differentiation from other respiratory infections
is difficult because the initial symptoms can be similar. Since the influenza virus is highly contagious, accurate diagnosis and
prompt treatment of patients can have a positive effect on public health. Rapid diagnosis of viral infection can also help
reduce the inappropriate use of antibiotics. Initiation of antiviral therapy for influenza within 48 hours of symptom onset
is recommended for quick improvement of symptoms and reduction in viral shedding.
4
The Silaris Influenza A&B Test is a
Nucleic Acid Amplification Test (NAAT). It is designed to be run at point of care locations and rapidly report detection of
influenza viruses A and/or B from patients.
PRINCIPLE OF THE TEST
The Silaris Influenza A&B Test is a point of care Nucleic Acid Amplification Test (NAAT) for detection of influenza A virus and/or
influenza B virus in patients with flu-like symptoms in approximately 30 minutes. To perform the test, nasal swab specimens are added
to the Nasal Swab Buffer to solubilize the sample. An aliquot of the Nasal Swab Buffer is then dispensed into an Silaris Influenza A&B
Test Cassette. The Test Cassette contains internal process positive and negative controls, enzymes, OscAR™ reagents, and a
detection strip necessary for the 4 steps in the assay. These 4 steps are lysis of the virus, reverse transcription of viral RNA to cDNA,
nucleic acid amplification, and detection. The Silaris Dock controls reaction temperatures, timing, and fluid movements within the Test
Cassette resulting in a fast and automated influenza A and influenza B assay. After approximately 30 minutes, the test results are
interpreted by the visualization of Blue Test Lines on the detection strip in the Test Cassette. A blue process control line at the control
(C) area is used to ensure proper reagent and Silaris™ Dock function and to confirm a valid negative test result.
REAGENTS AND MATERIALS
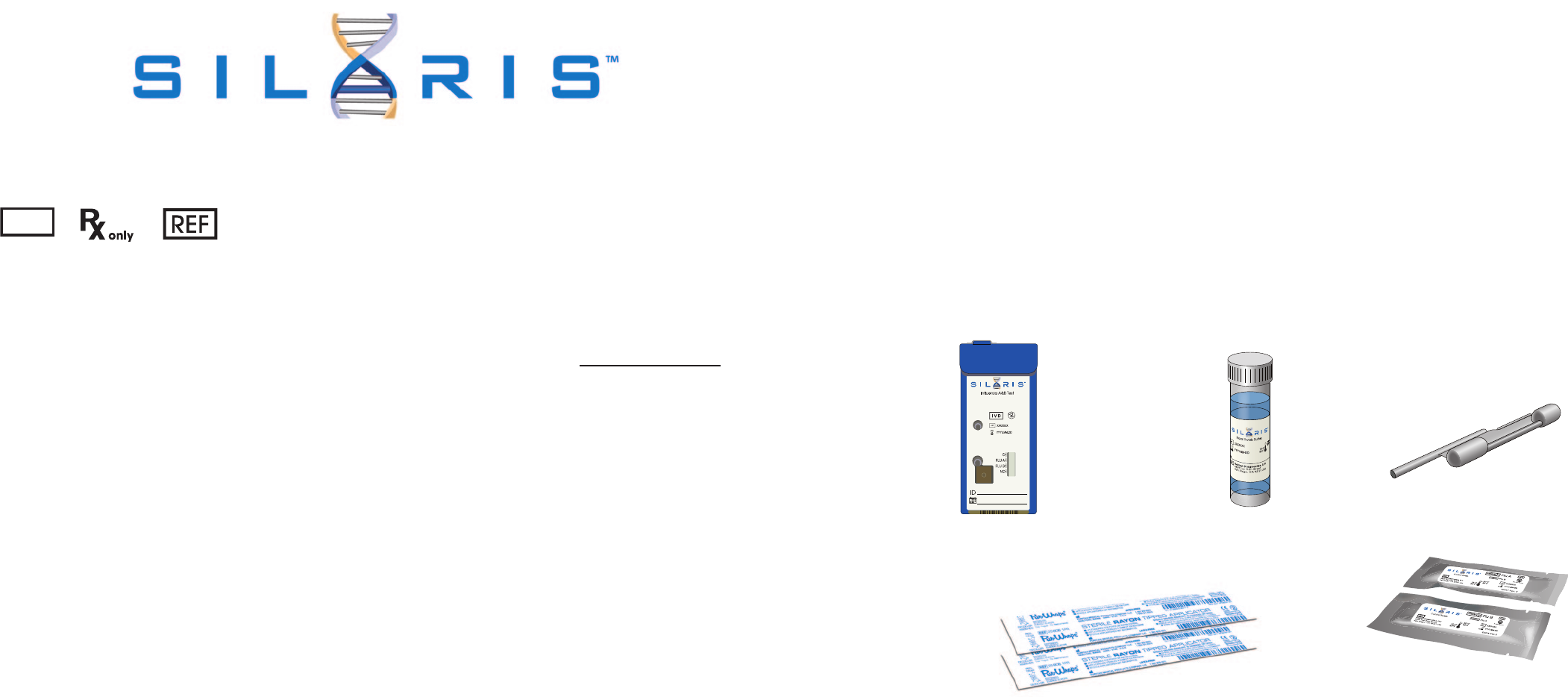
MATERIALS PROVIDED
Influenza A&B
Test Cassette
Nasal Swab Buffer
Transfer Pipette
Influenza A Positive Control Swab
Influenza B Positive Control Swab
All kit components must be stored at room temperature (15°C - 30°C, 59°F - 86°F).
SILARIS INFLUENZA A&B TEST CONTENTS:
• Rayon Swab (25): Sterile swab for nasal sample collection
• Nasal Swab Buffer (25): Single-use vial of solution containing 5mL of buffer with dimethyl sulfoxide and < 0.01% sodium azide.
• Transfer Pipette (25): Single-use, fixed volume Pipette used to transfer sample from the Nasal Swab Buffer vial into the Test
Cassette. NOTE: Supplied within the Test Cassette Pouch
• Silaris Influenza A&B Test Cassette (25): Single-use, foil-pouched with desiccant and Test Cassette containing lyophilized
reagents for the targeted amplification and detection of influenza A and B viral RNA.
• Control swab (1): Positive for Influenza A and negative for Influenza B (yellow shaft) . Contains inactivated influenza A virus dried
onto a swab.
• Control swab (1): Positive for Influenza B and negative for Influenza A (blue shaft). Contains inactivated influenza B virus dried
onto a swab.
• Instructions For Use (IFU) (1)
• Quick Reference Guide QRG (1)
INFLUENZA A&B TEST
P/N: 50033 Rev:0
Rayon Swabs
For use with the Silaris Dock
1 2










