Product Manual
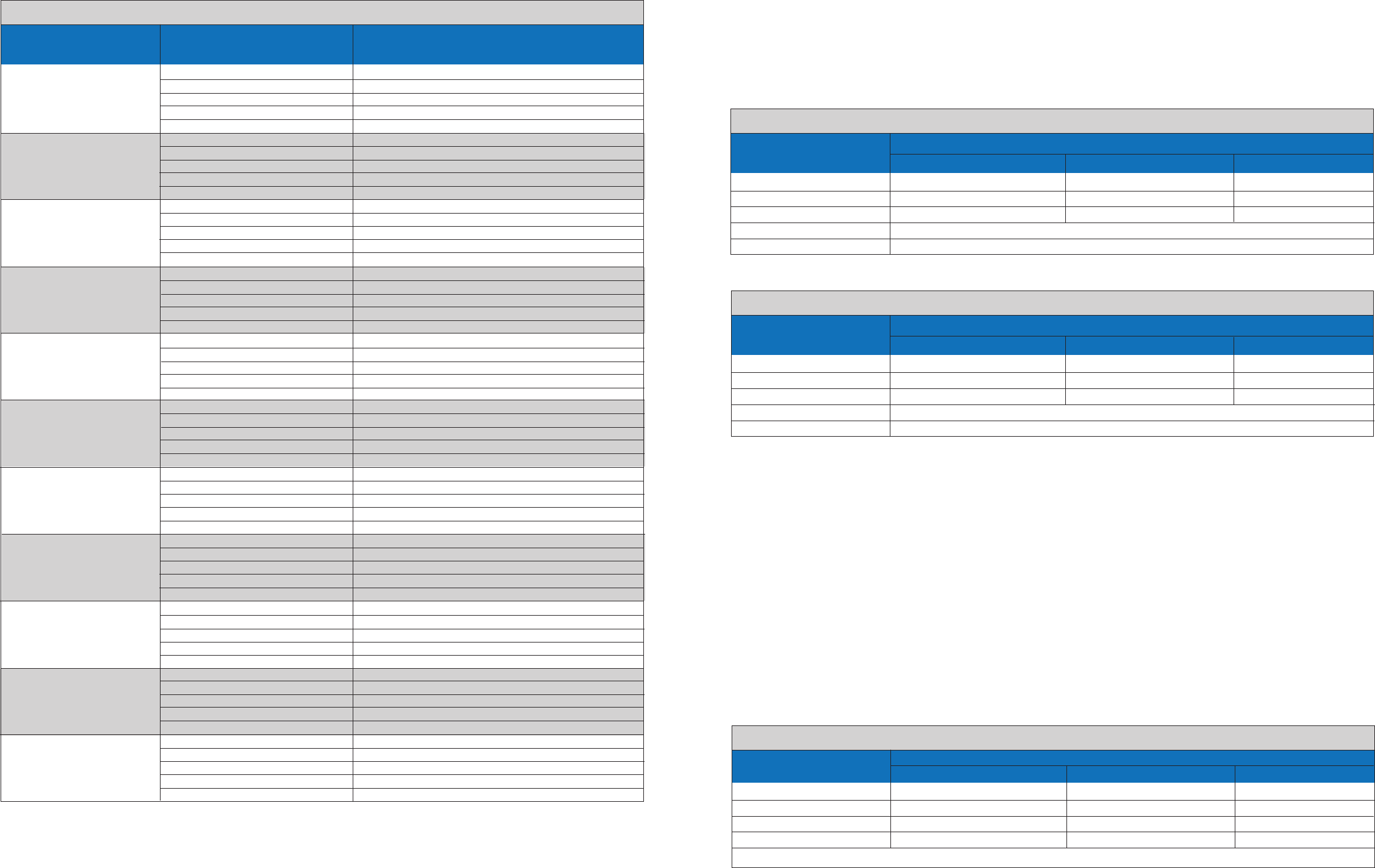
Interfering Substances: Agreement of Observed/Expected
Target % Agreement with Expected Results
Interferent Description,
Concentration
Mucin,
20 µg Mucin/mL
Blood (Human)
1% (v/v)
Neo-Synephrine
(phenylephrine nasal spray)
Negative 100% (3/3)
FluA/Cali 100% (3/3)
FluA/Texas 100% (3/3)
FluB/Nevada 100% (3/3)
FluB/Mass 100% (3/3)
Negative 100% (3/3)
FluA/Cali 100% (3/3)
FluA/Texas 100% (3/3)
FluB/Nevada 100% (3/3)
FluB/Mass 100% (3/3)
Negative 100% (3/3)
FluA/Cali 100% (3/3)
FluA/Texas 100% (3/3)
FluB/Nevada 100% (3/3)
FluB/Mass 100% (3/3)
Negative 100% (3/3)
FluA/Cali 100% (3/3)
FluA/Texas 100% (3/3)
FluB/Nevada 100% (3/3)
FluB/Mass 100% (3/3)
Negative 100% (3/3)
FluA/Cali 100% (3/3)
FluA/Texas 100% (3/3)
FluB/Nevada 100% (3/3)
FluB/Mass 100% (3/3)
Negative 100% (3/3)
FluA/Cali 100% (3/3)
FluA/Texas 100% (3/3)
FluB/Nevada 100% (3/3)
FluB/Mass 100% (3/3)
Negative 100% (3/3)
FluA/Cali 100% (3/3)
FluA/Texas 100% (3/3)
FluB/Nevada 100% (3/3)
FluB/Mass 100% (3/3)
Negative 100% (3/3)
FluA/Cali 100% (3/3)
FluA/Texas 100% (3/3)
FluB/Nevada 100% (3/3)
FluB/Mass 100% (3/3)
Negative 100% (3/3)
FluA/Cali 100% (3/3)
FluA/Texas 100% (3/3)
FluB/Nevada 100% (3/3)
FluB/Mass 100% (3/3)
Negative 100% (3/3)
FluA/Cali 100% (3/3)
FluA/Texas 100% (3/3)
FluB/Nevada 100% (3/3)
FluB/Mass 100% (3/3)
Negative 100% (3/3)
FluA/Cali 100% (3/3)
FluA/Texas 100% (3/3)
FluB/Nevada 100% (3/3)
FluB/Mass 100% (3/3)
Afrin
(Oxymetazoline nasal spray)
Nasacort (Triamcinolone,
nasal corticosteroid
No Interferent
Zicam
(Nasal gel, homeopathic
allergy relief medicine)
Cepacol
(throat lozenge)
Zanamivir
(anti-viral drug)
10mg/mL
Mupirocin (antibiotic)
12 mg/mL
Tobramycin (antibacterial)
2.43 mg /mL
CLIA WAIVER STUDIES
Clinical Performance by Intended Users
The performance of the Silaris Influenza A&B Test was evaluated at sixteen intended use sites by non-laboratory personnel in a prospective
clinical study during the 2016-2017 flu season in the United States. Nasal swabs were collected from patients with flu-like symptoms and
were tested with the Silaris Influenza A&B Test and the comparator method, a FDA-cleared molcular influenza assay. All specimens
generating discrepant results were investigated by testing using an alternative FDA-cleared molcular assay. The performance of the
Silaris Influenza A&B Test for influenza A and influenza B compared with the comparator method are presented in the tables below.
Silaris Influenza A&B Test Flu A performance against the Molecular Comparator Method
Silaris Influenza
A&B Test Flu A
289 60
a
349
9
b
900 909
298 960 1258
97% (95% CI: 94.4% - 98.4%)
94% (95% CI: 92.0% - 95.1%)
Comparator
Positive
Negative
Total
The study demonstrates the performance of the Silaris Influenza A&B Test in a CLIA Waived clinical setting.
Performance Near the Cut-off
Three CLIA-waived sites that participated in the prospective clinical study participated in the Near-Cutoff study. The testing was
performed by three (3) untrained intended operators at each of the sites. This study was conducted to demonstrate that untrained
intended users could perform the Silaris Influenza A&B Test and consistently detect Low Positive samples at the Limit of Detection.
The test panel consisted of three contrived samples: Flu A Low Positive, Flu B Low Positive, and a True Negative. Each sample was prepared
using Flu A and B strains spiked into clinical matrix. The Flu A strain used in this study was Flu A/California/07/2009 and the Flu B strain used in
this study was Flu B/Massachusetts/2/2012. The targeted concentrations for the Low Positive samples were approximately 1 X the respective
LoD (C95 concentration), and the Flu A and B Negative samples contained no Flu virus. Test samples of Influenza A or Influenza B were coded
and blinded to the operators. Swab specimens were presented to the intended use operators throughout the course of a normal testing day
and were masked as subject samples. Testing took place over the course of two weeks on non-consecutive days, while the clinical study was
in progress. Each operator tested 5 samples each testing day. Each site ultimately tested a panel of 60 samples: 20 replicates of each sample.
Testing was performed with one lot of Silaris Influenza A&B Test cassettes.
Test results are shown in the table below. This study demonstrates untrained intended use operators are able to accurately perform and
interpret the Silaris Influenza A&B Test at the level of the LoD for both Influenza A and Influenza B.
Positive
Negative
Total
Sensitivity:
Specificity:
a
FLU A was detected in 47/60 False Positives specimens using an alternative FDA-cleared molecular Influenza Assay
b
FLU A was not detected in 3/9 False Negative specimens using an alternative FDA-cleared molecular Influenza Assay
Silaris Influenza A&B Test Flu B performance against the Molecular Comparator Method
Silaris Influenza
A&B Test Flu B
126 14
a
140
8
b
1110 1118
134 1124 1258
94% (95% CI: 88.7% - 97.0%)
99% (95% CI: 97.9% - 99.3%)
Comparator
Positive
Negative
Total
Positive
Negative
Total
Sensitivity:
Specificity:
a
FLU B was detected in 9/14 False Positives specimens using an alternative FDA-cleared molecular Influenza Assay
b
FLU B was not detected in 5/8 False Negative specimens using an alternative FDA-cleared molecular Influenza Assay
Near-Cutoff Study Test Results: Agreement of Observed/Expected
ADP 19/20 19/20 19/19*
DCO 20/20 20/20 20/20
GVP 19/20 19/20 20/20
Total Agreement 58/60 = 97% 58/60 = 97% 59/59 = 100%
Low A Positive/Total
Low B Positive/Total
Negative/Total
Site
*1 negative result resulted in an unresolved Invalid (2 invalid results on the same sample)
Swab Type
19
20










