Product Manual
Interfering Substances
To assess substances with the potential to interfere with the performance of the Silaris Influenza A&B Test, four (4) influenza strains
were tested in replicates of three (3) with each interfering substance at the “worst case” concentration. The influenza strains selected
for testing include a 2009 pandemic swine-like H1N1 influenza A strain, an H3N2 influenza A strain, and two influenza B strains
representing Victoria and Yamagata lineages. Virus was serially diluted into a pooled clinical matrix to achieve a 1.5X LoD concentration.
Each influenza strain was tested with the “worst case” interferent concentration, representing the highest concentration likely to be
found in a respiratory sample. Additionally, each strain was tested without the interfering substance as a control.
The results are shown in the table. The Silaris Influenza A&B Test performance is not negatively affected by the potentially interfering
substances under “worst case” concentration conditions.
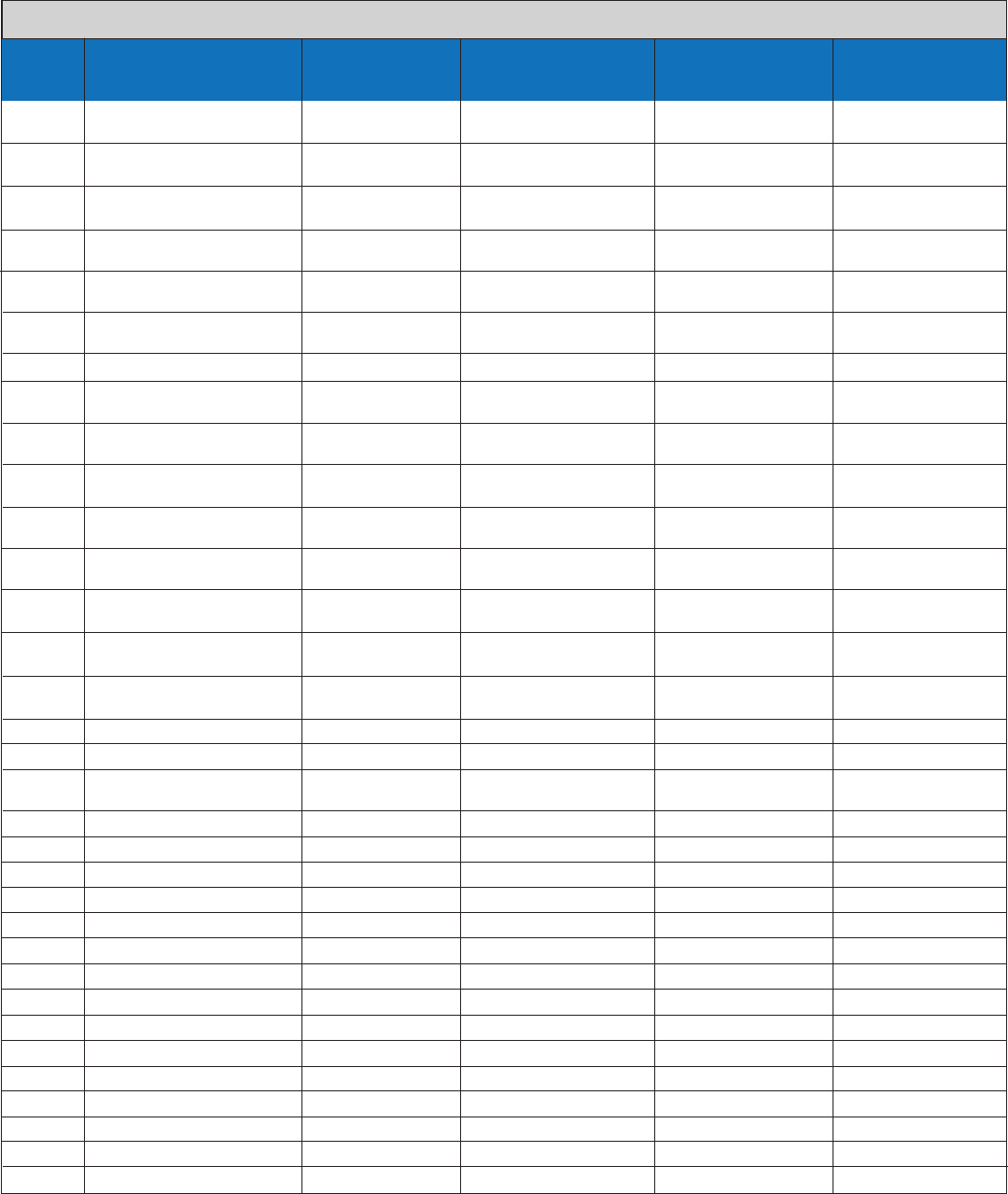
Analytical Exclusivity – Organisms Tested, Concentrations and Test Results
Organism
Key #
1 Adenovirus Type 1 1.02E+08 5.10E+05 TCID50/mL 0/3 0/3
TCID50/mL
2 Adenovirus Type 7 6.61E+06 3.31E+04 TCID50/mL 0/3 0/3
TCID50/mL
3 Human herpesvirus 5 2.19E+06 1.10E+04 TCID50/mL 0/3 0/3
(Cytomegalovirus) TCID50/mL
4 Human coronavirus 229E 2.19E+06 1.10E+04 TCID50/mL 0/3 0/3
TCID50/mL
5 Human coronavirus OC43 5.89E+07 2.95E+05 TCID50/mL 0/3 0/3
TCID50/mL
6 Human Enterovirus 71 4.17E+05 1.04E+04 TCID50/mL 0/3 0/3
(HEV-71) TCID50/mL
7 Epstein-Barr virus 7.95E+09 cp/mL 3.98E+07 cp/mL 0/3 0/3
8 Human parainfluenza 1.26E+06 1.26E+04 TCID50/mL 0/3 0/3
virus 1 TCID50/mL
9 Human parainfluenza 2.19E+06 1.10E+04 TCID50/mL 0/3 0/3
virus 2 TCID50/mL
10 Human parainfluenza 5.89E+05 1.18E+04 TCID50/mL 0/3 0/3
virus 3 TCID50/mL
11 Measles virus 5.89E+07 2.95E+05 TCID50/mL 0/3 0/3
TCID50/mL
12 Human metapneumovirus 3.55E+05 1.01E+04 TCID50/mL 0/3 0/3
TCID50/mL
13 Mumps virus 1.95E+07 9.75E+04 TCID50/mL 0/3 0/3
TCID50/mL
14 Respiratory syncytial virus 3.16E+06 1.58E+04 TCID50/mL 0/3 0/3
TCID50/mL
15 Human rhinovirus 17 6.61E+06 3.31E+04 TCID50/mL 0/3 0/3
TCID50/mL
16 Bordetella pertussis 8.43E+08 cfu/mL 4.22E+06 cfu/mL 0/3 0/3
17 Chlamydia pneumoniae ≥ 5E+03 IFU/mL* ≥ 1.67E+04 IFU/mL 0/3 0/3
18 Corynebacterium ≥ 5.56E+07 ≥ 1.59E+06 IFU/mL 0/3 0/3
glycinophilum IFU/mL**
19 Escherichia coli 3.83E+09 cfu/mL 1.92E+07 cfu/mL 0/3 0/3
20 Haemophilus influenzae 2.40E+08 cfu/mL 1.20E+06 cfu/mL 0/3 0/3
21 Lactobacillus sp. 6.00E+08 cfu/mL 3.00E+06 cfu/mL 0/3 0/3
22 Legionella longbeachae 1.93E+09 cfu/mL 9.65E+06 cfu/mL 0/3 0/3
23 Moraxella catarrhalis 3.97E+07 cfu/ml 1.99E+05 cfu/ml 0/3 0/3
24 Mycobacterium tuberculosis 7.23E+08 cfu/mL 3.62E+06 cfu/mL 0/3 0/3
25 Mycoplasma pneumoniae 5.62E+07 CCU/mL 2.81E+05 CCU/mL 0/3 0/3
26 Neisseria meningitidis 2.55E+08 cfu/mL 1.28E+06 cfu/mL 0/3 0/3
27 Neisseria subflava 1.46E+09 cfu/mL 7.30E+06 cfu/mL 0/3 0/3
28 Pseudomonas aeruginosa 1.21E+08 cfu/mL 6.05E+05 cfu/mL 0/3 0/3
29 Staphylococcus aureus 1.39E+10 cfu/mL 6.95E+07 cfu/mL 0/3 0/3
30 Staphylococcus epidermidis 6.47E+09 cfu/mL 3.24E+07 cfu/mL 0/3 0/3
31 Streptococcus pneumonia 4.17E+08 cfu/mL 2.09E+06 cfu/mL 0/3 0/3
32 Streptococcus pyogenes 5.43E+09 cfu/mL 2.72E+07 cfu/mL 0/3 0/3
33 Streptococcus salivarius 4.63E+08 cfu/mL 2.32E+06 cfu/mL 0/3 0/3
Organism Name
Stock
Concentration
Test Level
The Analytical Specificity (Cross-Reactivity)
The analytical specificity was evaluated with a panel of common organisms when tested on the Silaris Influenza A&B Test. Thirty-three
(33) organisms were obtained from Zeptometrix Corporation except for Chlamydia pneumonia and Corynebacterium glycinophilum
(were obtained from ATCC). These potentially cross-reacting non-influenza organisms were tested in replicates of three (3) in this study.
The organisms were diluted into a Pooled Negative Nasal Sample (PNNS) matrix to create samples at the concentration in the table
below for testing.
Flu B Test Result
(# of FluB Positive /3)
Flu A Test Result
(# of FluA Positive /3)
All 33 exclusivity organisms were negative at the concentrations tested. Exclusivity is verified for the strains tested.
17
18










