Datasheet
www.recom-power.com
REV.: 1/2019 MED-5
DC/DC Converter
Specifications (measured @ Ta= 25°C, nom. Vin, full load and after warm-up unless otherwise stated)
REM6E
Series
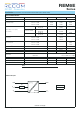
-40 -20 0 20 40 7060-30 -10 10 30 50 80
9575 85
90
110
100
100
80
60
40
90
70
50
30
20
10
0
Output Load [%]
Ambient Temperature [°C]
Derating Graph
(@ Chamber and natural convection 0.1m/s)
SAFETY AND CERTIFICATIONS
Certificate Type (Safety) Report / File Number Standard
Medical Electric Equipment, General Requirements for Safety and
Essential Performance
E314885
CAN/CSA-C22.2 No. 60601-1:14, 3rd Edition: 2014
ANSI/AAMI ES60601-1:2012
Medical Electric Equipment, General Requirements for Safety and
Essential Performance
pending EN60601-1:2006 + A12:2014
Medical Electric Equipment, General Requirements for Safety and
Essential Performance (CB Scheme)
pending IEC60601-1:2005, 3rd Edition + AM1:2012
RoHS 2+ RoHS 2011/65/EU + AM2015/863
EMC Compliance
Condition
Standard / Criterion
Medical electrical equipment Part 1-2: Electromagnetic
disturbances – Requirements and tests
pending IEC60601-1-2
Information technology equipment - Radio disturbance
characteristics - Limits and methods of measurement
with external filter EN55032, Class A and B
ESD Electrostatic discharge immunity test Air ±15kV, Contact ±8kV EN61000-4-2, Criteria A
Radiated, radio-frequency, electromagnetic field immunity test
10V/m EN61000-4-3, Criteria A
Fast Transient and Burst Immunity
DC Power Port: ±2kV EN61000-4-4, Criteria A
Surge Immunity
DC Power Port: ±1kV EN61000-4-5, Criteria A
Immunity to conducted disturbances, induced by radio-frequency fields
10Vr.m.s EN61000-4-6, Criteria A
continued on next page