Manual
IKA
-WERKE C 5000 control/duo-control Ver. 10 04.07
3DJH
&DORULPHWULFPHDVXUHPHQWV
'HWHUPLQLQJWKHJURVVFDORULILFYDOXH
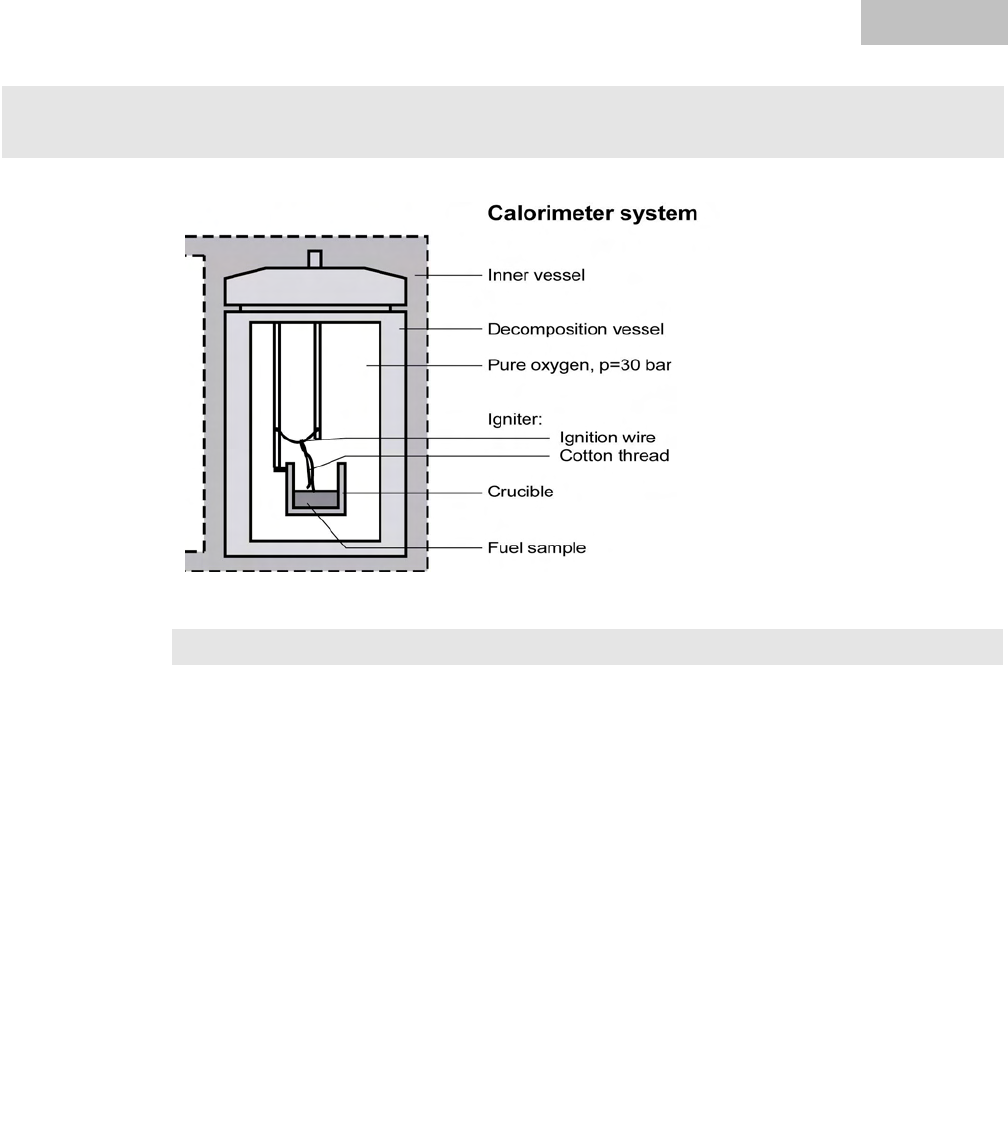
In a calorimeter, combustion processes take place under precisely defined condi-
tions. For this purpose, the decomposition vessel is charged with a weighed in fuel
sample, the fuel sample is ignited, and the increase in temperature in the calorime-
ter system is measured. The specific gross calorific value of the sample is calculated
from:
• the weight of the fuel sample
• the heat capacity (C value) of the calorimeter system
• the increase in temperature of the water in the inner vessel of the measurement
cell
To optimize the combustion process, the decomposition vessel is filled with pure
oxygen (99.95 %). The pressure of the oxygen atmosphere in the decomposition
vessel is 30 bar.
The exact determination of the gross calorific value of a substance is based on the
requirement that the combustion proceeds under precisely defined conditions. The
applicable standards are based on the following assumptions:
• The temperature of the substance to undergo combustion is 22°C before com-
bustion.
• The water contained in substance and the water formed during combustion of
compounds in the substance containing hydrogen are present after combustion
in liquid state.
• No oxidation of atmospheric nitrogen takes place.
• The gaseous products of combustion consist of oxygen, nitrogen, carbon diox-
ide and sulfur dioxide.
• Solid ash is formed.
&DORULPHWH U
V\VWHP
([SHULPHQW
FRQGLWLRQV










