Instruction manual
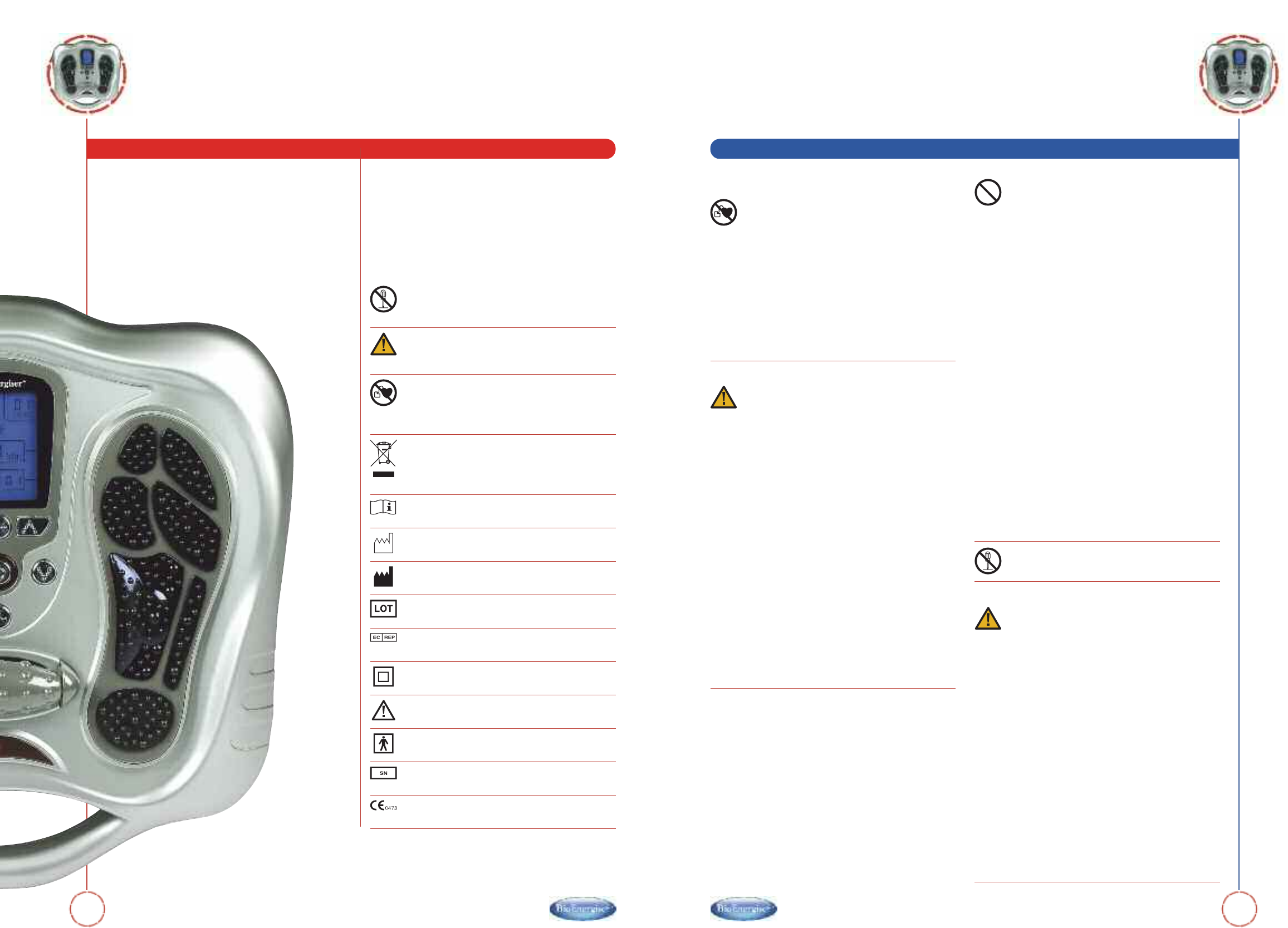
Notes on Safety
The icons and warning signs are indicated here for your
safety and correct usage of the product as well as to
prevent injuries and/or damage to properties.
The icons and meanings are as follows:
Examples of Icon
This icon means prohibited (must not do).
Anything prohibited is marked clearly with this
warning symbol.
This icon indicates something that is compulsory
(must be observed). Compulsory actions are
marked clearly with this warning symbol.
This product should not be used by persons with
electronic medical implants, e.g. heart
pacemakers, organ transplant or other electronic
life support systems.
Disposal of this product and used batteries
should be carried out in accordance with the
national regulations for the disposal of electronic
products. Directive 2002/96/EC(WEEE).
Consult instructions for use
Date of manufacturer
Manufacturer’s name
Batch Code
Authorised representative in the European
Community.
Class II equipment
Caution, consult accompanying documents
Type BF applied Part
This symbol means serial number which is on the
underside of the device and on the packaging.
CE Mark: conforms to essential requirements of
the Medical Device Directive 93/42/EEC
Danger
This unit must not be used in combination with
the following me
dical d
evices:
• Internally transplanted electronic medical devices, e.g.
pacemakers
• Electronic life support equipment, such as respirators
• Electronic medical devices attached to the body, such
as electrocardiographs
Using this unit with other electronic medical
devices may cause erroneous operation of those
devices.
Warning
Persons with the following conditions must
consult the doctor before using this unit:
• Acute disease
• Malignant tumor
• Infectious disease
• Pregnancy
• Cardiac dysfunction
• High fever
• Abnormal blood pressure
• Skin sensory disorders or skin problems
• Receiving medical treatment, especially those
feeling discomfort
Should not be used by persons in the first
trimester of pregnancy, fitted with a pacemaker
or AICD, o
r b
eing treated for an existing deep
vein thrombosis
Do not use this unit near the heart, above the neck,
on the head, around the mouth or on diseased skin.
Application of electrodes near the thorax may
increase the risk of cardiac fibrillation.
Do not use this unit simultaneously with other
therapeutic devices or in combination with
ointments including spray type ointments.
Simultaneous connection of a PATIENT to h.f.
surgical EQUIPMENT
may r
esult in burns at the
site of the STIMULATOR electrodes and possible
damage to the STIMULATOR.
Operation in close proximity (e.g. 1 m) to a
shortwave or microwave therapy EQUIPMENT
may produce instability the STIMULATOR output.
Do not use this unit for purposes other than
treatment indicated in this manual. May lead to
accident, problems, or failure of the unit.
Do not insert the electrode cord plug into any
place other than the electrode cord jack of the
main unit. May ca
use a
n electric shock or
accident.
Do not disassemble or remodel this unit.
Caution
If you feel any problems with your body or skin,
consult your medical practitioner and follow their
instructions.
If not, you may
receive a strong electrical shock.
You may receive
strong electrical shock.
The settings and timings of
the device may be affected.










