Use Instructions
Table Of Contents
- GLOSSARY & ACRONYMS
- PART 1. GENERAL SYSTEM INFORMATION
- 2. INDICATIONS AND CONTRAINDICATIONS FOR USE
- 3. WARNINGS AND PRECAUTIONS
- CAUTION
- 6. BASELINE MEASUREMENT PREPARATION AND DEVICE SETUP
- 8. THE ALIGNMENT TOOL
- PART 3. AT-HOME CPM SYSTEM USE
- PART 4. LIGHTS, SOUNDS, AND TROUBLESHOOTING
- 12. CPM DEVICE LIGHTS AND SOUNDS
- 13. BASE STATION INDICATIONS
- 14. BUTTON PRESSES
- 15. GENERAL TROUBLESHOOTING / FAQS
- 15.1 Physician Q&As
- Q: No data from my patient is appearing on the server, but after getting in touch with the patient, they say that they have been taking measurements consistently with no issue and their device is connected to a plugged- in Base Station.
- Q: No size of the CPM Device fits my patient.
- Q: I initiated a patient mode measurement while trying to put the device into Bluetooth pairing mode and now I can’t pair the CPM Device to the Mobile App.
- Q: How do patients get more adhesives when they run out?
- Q: Can the CPM System be used near other electronic or medical equipment such as telephones, televisions, computers, etc.?
- Q: Is data secure when transmitting from the CPM Device to the Mobile App, the Device to the Base Station, or the Base Station to the Cloud platform?
- 15.2 Patient Q&As
- Q: The CPM Device is not lighting up when plugged into the Base Station to charge.
- Q: Nothing happens when I am trying to start a measurement and/or the button is pressed on the CPM Device.
- Q: The device visually does not stick well to my body or is obviously peeling up.
- Q: I lost the clear protective liners for covering the adhesive.
- Q: I pressed the button accidentally/too early and a measurement started before the device was on my body. How do I get the device to stop beeping?
- Q: The CPM Device continues to beep, indicating there are errors, during a measurement.
- Q: Can I shower with the CPM Device or get it wet?
- Q: Can I travel with the CPM System?
- Q: A piece or part broke off the device, base station, or alignment tool.
- Q: What do I do if I think I am having a medical emergency or decompensation event?
- 15.1 Physician Q&As
- PART 5. CLEANING AND MAINTENANCE
- PART 6. SYSTEM SPECIFICATIONS
- PART 7. SYMBOLS GLOSSARY
23
Version C
Clinician Instructions for Use – Device and Mobile Application
6.2 Preparing the skin
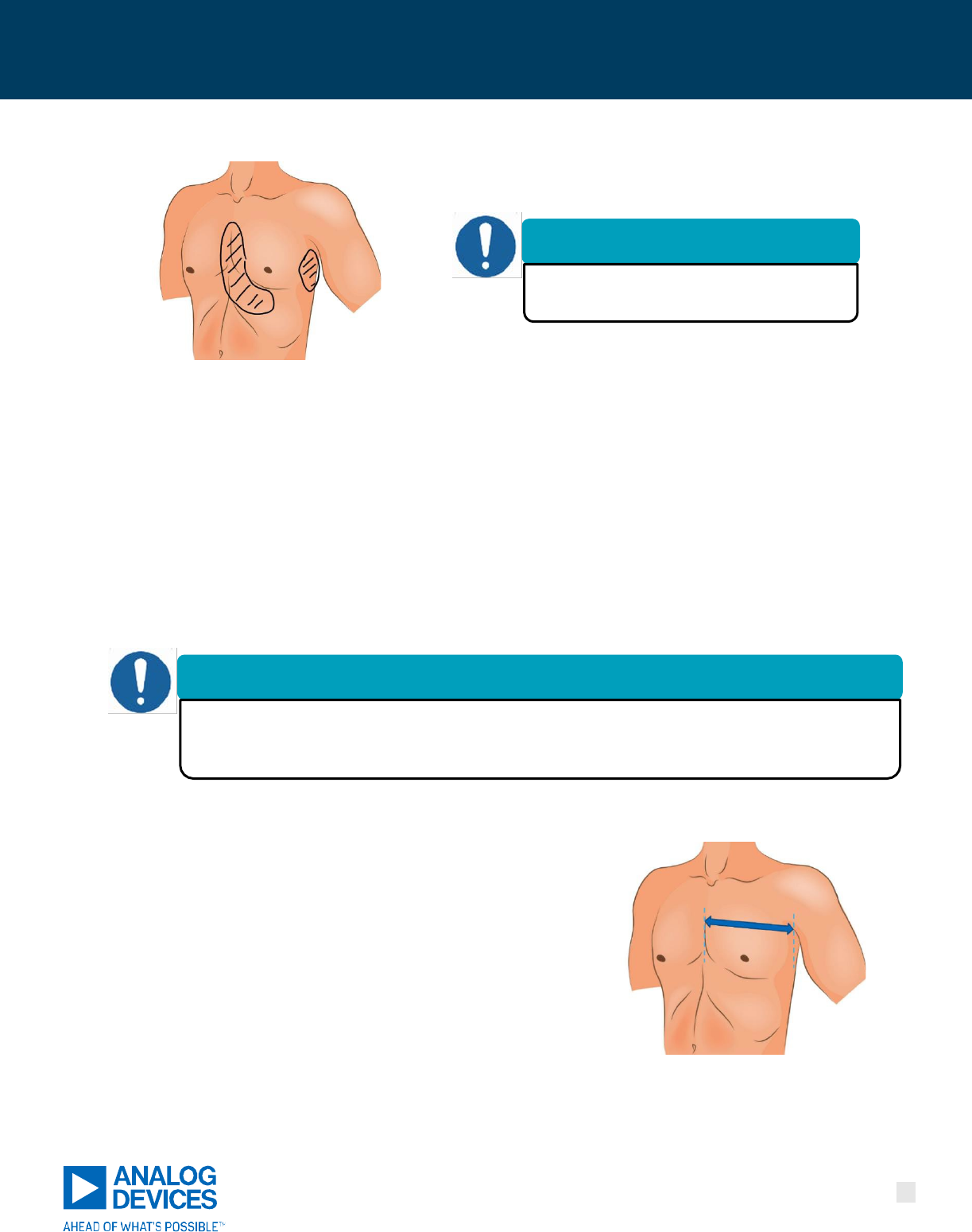
Figure 13. Areas to be trimmed under the CPM Device.
•
Trim chest hair where the CPM Device adhesive patches contact the body (about a 6” long x 2” wide area at
the sternum, under the left nipple, and a 4” long x 2” wide area on the left side of the chest).
Trimming may not be needed in all cases. If baseline data collection proves to be an issue, it may be
necessary to remove the CPM Device, trim, and retry placing the device.
Patients may have to periodically trim hair at home depending on their length of device use.
In certain cases, patients may be instructed by clinicians to wet the skin in the area under the device
electrodes with a damp cloth before placing the CPM Device, especially in cases of extremely dry skin.
Clinicians should give these instructions to the patient only if they are having severe difficulties with
electrode contact errors during the Baseline measurement that cannot be resolved by pressing down
on the electrodes, replacing the device, or trimming hair.
6.3 Fitting and sizing the CPM Device
1)
Extend the CPM Device to the desired length by holding the
device up to the patient’s body and gently pulling the flexible
material out of the rigid housing in the middle of the device.
You can use a tape measure if desired. The device should
extend to the anterior- or mid-axillary line on the left side of
the patient’s chest.
2)
Before locking this position permanently in place, hold the
device to the patient’s chest to ensure that the measured
size is appropriate (i.e. not too long or short, as illustrated
below).
Figure 14. Distance to be measured for CPM
Device sizing.
IMPORTANT
This step ensures good adhesion to the
skin and improves data quality.
IMPORTANT
NO
creams or lotions should be applied to the skin and skin should be dry of water immediately
prior to the application of the device except for special circumstances.
Patients should follow
any special directives given by the Clinical Care Team for these circumstances.










